How do cancers and tumours evade the immunity? Current therapeutic options and future research
Cancer is one of the deadliest diseases causing very high death toll every day in the world. Frequently, cancer has a poor prognosis. Multiple therapeutic strategies and treatment options have been developed for various cancers and tumours. The chemotherapy, radiotherapy, targeted therapy, and immunotherapy are applied over the past several decades. However, resistance to these therapies is a major issue that impedes the clinical outcomes and effectiveness of cancer treatments and impacts patient survival. The patients with early stage positively respond to the treatments, but they poorly respond and show dire clinical outcomes at a later stage (Wu et al., 2021 and references therein).
Tumours, Structure and Immune System
The tumours produce tumour antigens that can enable the immune system to differentiate tumour cells from normal cells. Antigens trigger the immune response and activate the T cell response that can provide defense against tumourigenesis. But tumour cells develop some mechanisms to evade or avoid host immunity for their growth and survival (Spurrell and Lockley, 2014).
Tumours grow within a complex environment of structures, cells, and chemical signals ranging from epithelial cells, stroma, blood, and lymphatic vasculature, immune cells, cytokines, and chemokines. The structure of a typical tumour consists of the tumour core, the invasive margin, and the surrounding stromal and lymphoid components. Some components within the immune milieu are beneficial, while some components are deleterious to the patient. There are different immune cells identified in different locations within a tumour. The clinical outcome is presumed to be affected due to the variation in density and distribution of immune cells within tumours. The anti-tumour immunity can be increased by the down-regulation of immune checkpoint proteins. The antibodies are increasingly used to up-regulate host anti-tumour immunity with some durable results seen in early trials so far (Spurrell and Lockley, 2014).
What is Tumour Microenvironment?
The tumour microenvironment (TME) is the extracellular environment that contains tumours cells, carcinogenetic cells, cancer-associated fibroblast (CAFs), immune cells, the vasculature system, and the extracellular matrix (ECM) including secreted cytokine, chemokine, metabolites, and exosomes. The genetic and epigenetic alterations in tumour cells provide them active resistance capacity. Besides this, the tumour microenvironment (TME) plays a key role in tumorigenesis, metastasis, progression, and adaptive resistance to cancer treatment (Wu et al., 2021 and references therein).
Adaptive Mechanisms of Cancer/Tumour Resistance
Wu et al. (2021) indicated that the adaptive mechanisms of tumour resistance are intimately related to tumour microenvironment. However, adaptive mechanisms driven by the TME are not yet clearly understood and need detailed studies to fully elucidate the mechanisms of tumour therapeutic resistance. But there are some evidences that many clinical treatments targeting the TME have been successful. Cancer cells escape from the cytotoxicity of tumour therapies through the following intrinsic mechanisms, e.g., decreased drug accumulation, altered drug metabolism, mutated, or altered drug target and enhanced DNA repair capability, as well as inactivated cell death signalling. Tumour cell heterogeneity is one of the factors that creates various resistance responses for multiple therapies. Sometimes, tumours progression continues, even though they face external pressure from various therapies. Therefore, new theories have proposed that tumour progression is a dynamic and complicated process that tightly interacts with the surrounding environment (Wu et al., 2021 and references therein).
The non-malignant cells in the TME actively boost carcinogenesis by promoting excessive tumour initiation, malignant progression, metastasis, and therapeutic resistance. The transformed cancer cells contribute extensively to tumour development and resistance by interaction with stromal cells in the TME. Some preclinical studies indicate that the TME is a potential therapeutic target (Jin and Jin, 2020). For instance, multiple strategies of combined therapy related to the TME have shown interesting potential (Wu et al., 2021 and references therein).
Tumours and Immunity Evasion
The development of tumours despite host immunity indicates that tumour cells develop immune avoidance. Tumours develop immunity resistance through several mechanisms (Wu et al., 2021 and references therein):
- Some tumours have been demonstrated to lose expression of MHC molecules making them unable to present tumour antigens, thus evading T cell recognition.
- Some tumours secrete immunosuppressive cytokines, e.g., IL-10.
- Some tumours generate physical barriers, e.g., collagen and fibrin, thus making them invisible to the immune system.
- Tumours can also evade the immune response by up-regulating inhibitory molecules and inducing a form of self-tolerance.
- An immune response can be produced by T cells depending on co-stimulatory signals. However, inhibition of co-stimulatory signals creates immune tolerance. Cytotoxic T-lymphocyte associated antigen 4 (CTLA4) and programmed cell death protein 1(PD1) are both inhibitory receptors involved in down-regulation of immune responses.
Tumour Immunology, Antibody, and Clinical Outcome
The level of immune cell infiltration correlates with prognosis in many cancers. Different populations of cells within the immune infiltrate affect prognosis in different ways. Monoclonal antibodies can target different types of tumour antigens for both solid tumours and haematological malignancies. Antibodies can kill tumour cell through several mechanisms (Wu et al., 2021 and references therein).
- Direct action of the antibody: the binding of antibody to the cell causes receptor blockade that inhibits the downstream signalling pathways within the cell, ultimately leading to apoptosis.
- Immune-mediated mechanisms include antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC).
- The inhibition of immune checkpoint proteins by targeted antibodies, e.g., anti-CTLA4 and anti-PD1 can do the immune modulation of T cell function.
- Conjugation of a radioisotope to antibody is used as a therapeutic in lymphoma.
- Many clinical trials combining anti-PD (programmed cell death) therapy with other antitumour drugs produced no synergistic or additive effect. Therefore, Kim et al. (2022) emphasized that defining adaptive immune resistance mechanisms at the tumour site should be a key focus to direct future drug development as well as practical approaches to improve current cancer therapy.
What is Immunotherapy
Immunotherapy is one kind of cancer treatment strategies that boosts the patient’s own immune system to eliminate cancer cells in solid tumours (Xu et al., 2022). More than 70 FDA-approved immunotherapy drugs were available up to now at April 2022. A plenty of immunotherapy-related clinical trials have been registered around the world for more than 50 types of cancers. Immunotherapy mainly includes cancer vaccines therapy, oncolytic virus therapy, dendritic cell (DC) therapy, adoptive cell therapy, antibody–drug conjugates (ADCs), and immune checkpoint inhibitors (ICIs). Out of these, ICIs, which use antibodies to programmed cell death protein 1 (PD-1) and programmed death ligand 1 (PD-L1) showed the most success (Xu et al., 2022). Although new technologies single-cell multi-omics may solve many problems, cancer immunotherapy still faces many challenges.
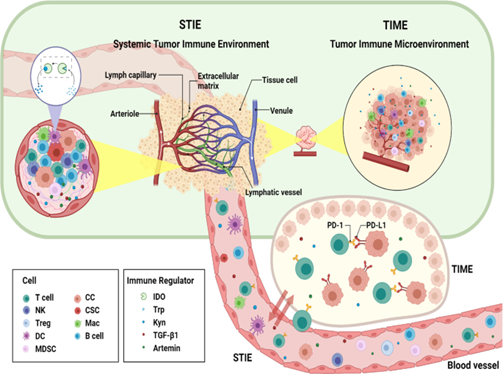
What is Tumour Immune Microenvironment?
Solid tumour tissue consists of highly heterogeneous cancer cells (Xu et al., 2022). The different upstream mutations and the tumour microenvironment (TME) form the variable compositions and evolutionary states of these cancel cells. The composition of TME controls the tumour–host interaction. Tumour immune microenvironment (TIME), compositing different cell groups of the immune system and their interactions in the TME niche significantly control the processes of carcinogenesis, cancer progression, and responses to the treatments (Xu et al., 2022).
What is Systemic Tumour Immune Environment (STIE)?
Systemic tumour immune environment (STIE) contains the immune modulating molecules and immune cells and mainly governed by the circulating blood and lymphatic vessels (Xu et al., 2022). It has significant role in communication between the primary tumour site to the distant organs and the host immune organs such as bone marrow and lymph nodes. The functional immune modulators are proteins, cytokines, and metabolites, while the immune cells comprise myeloid cell lineages (neutrophils, monocytes, megakaryocytes, platelets, basophils, eosinophils) and lymphoid cell lineages (T cells, B cells/plasma cells, and NK cells).
Relationship and Interactions between TIME and STIE
There are extensive interactions between tumour immune microenvironment (TIME) and systemic tumour immune environment (STIE). Investigation on the TIME of tumour in situ and metastatic sites, along with STIE in blood, plays a significant role to understand the mechanism of tumour progression and overall cancer treatment (Xu et al., 2022). Looking for the key factors in STIE and TIME may identify pathway to improve the treatment efficiency of PD-1 inhibitors. As more clinical trials continue, effective combination of radiotherapy and immunotherapy will continue to make great breakthroughs.
Multiple Strategies to Prevent Immune Resistance
Multiple strategies targeting the TME have been investigated to prevent resistance to radiotherapy, chemotherapy, and immunotherapy (Wu et al., 2021 and references therein).
- Cancer-Associated Fibroblasts (CAFs) is the most abundant cell type in the TME that have the critical role in therapeutic resistance of tumour cells. Therefore, targeting CAF functions is a promising approach for tumour therapy.
- It has been shown to be an efficient tumour therapy strategy to stop the communication between tumour cells and their environment by targeting adhesion molecules, proteolytic enzymes, and ECM components.
- Different strategies can be used to target the immune system in TME and tackle cancers, because the immune system in TME influences the response of tumours to various clinical therapies.
- The low oxygen pressure and acidosis conditions in the TME affect a tumour’s response to treatment. Therefore, manipulating hypoxia and acidosis conditions in the TME can stop tumour progression.
- Capturing and leading the TME to increase drug delivery can significantly increase the efficacy of chemotherapeutic drugs.
Recommendations
Although manipulating the TME has shown success to overcome resistance to cancer/tumour treatments, most of these studies used the resistant cells in vitro (model study) (Wu et al., 2021 and references therein).
- Further research is needed in a real TME to understand the mechanisms underlying adaptive and non-adaptive resistance.
- The factors involved in the real TME might be crucial for acquired resistance in vivo.
- Simulate a real TME consisting of tumour cells and other types of cells and tumour-microenvironment-on-a-chip (TMOC) models can only partially mimic the real TME.
- An appropriate mouse models, or human organoids isolated from patient biopsies can be used to fully understand the role of TME.
- Monitoring the factors controlling the therapeutic effects of targeting the TME can improve the success of different therapies and clinical outcomes.
Reference
Kim T.K., Vandsemb E.N., Herbst R.S. et al., 2022. Adaptive immune resistance at the tumour site: mechanisms and therapeutic opportunities. Nat Rev Drug Discov 21, 529–540.
Spurrell E.L, Lockley M., 2014. Adaptive immunity in cancer immunology and therapeutics. ecancer https://doi.org/10.3332/ecancer.2014.441
Wu P, Gao W, Su M, Nice EC, Zhang W, Lin J, Xie N, 2021. Adaptive Mechanisms of Tumor Therapy Resistance Driven by Tumor Microenvironment. Front. Cell Dev. Biol. 9:641469.
Xu, L., Zou, C., Zhang, S. et al., 2022. Reshaping the systemic tumor immune environment (STIE) and tumor immune microenvironment (TIME) to enhance immunotherapy efficacy in solid tumors. J Hematol Oncol 15, 87. https://doi.org/10.1186/s13045-022-01307-2
Prostate Enlargement
Dr Md Anawar Hossain
What is Benign Prostatic Hyperplasia (BPH)?
Benign prostatic hyperplasia (BPH) is defined as the prostate enlargement. The prostate grows and gets larger as a man ages. Prostate enlargement causes problems when it is large enough. Generally, the size of prostate is equal to that of a walnut or golf ball in adult men, but it can grow to be as large as an orange.
Enlargement of prostate gland causes following problems:
- It can squeeze the urethra,
- Bladder wall becomes thicker,
- Bladder may weaken over time and can’t empty urine fully,
- Urine then remains in the bladder,
- Result in lower urinary tract symptoms,
- Immediately consult your doctor if you can’t pass urine at all or if you have renal failure.
Common signs and symptoms
- The severity of symptoms varies from men to men depending on the severity of their individual prostate gland enlargement. Therefore, please consult your doctor if you feel you have the following symptoms, because the symptoms tend to gradually worsen over time.
- The prostate enlargement can bother or block the bladder,
- Frequent urination is a common symptom,
- Urination might occur every 1 to 2 hours, mainly at night,
- Men can’t empty the urine and feels that the bladder is still full and needs urination,
- Feeling the urgent need to urinate,
- Need to stop and start several times when passing urine,
- Frequency of urination at night, more than two times,
- Difficulty in passing urine at the beginning,
Severe cases:
- Inability to urinate when benign prostatic hyperplasia becomes severe,
- Infection in urinary tract and leading to bladder damage,
- Blood in the urine,
- Causing kidney damage.
Is it cancer: Benign Prostatic Hyperplasia (BPH)?
- No, it is not cancer,
- It is benign,
- But BPH and cancer can happen at the same time,
- Treatment can help to relieve symptoms,
- BPH is common and found in about half of all men between ages 51 and 60,
- Up to 90% of men over age 80 have it.
Causes of Benign Prostatic Hyperplasia (BPH)
Benign prostatic hyperplasia mainly occurs in older men. Although the causes are not clear, hormone changes are thought to play a role in benign prostatic hyperplasia. Hormones from the testis may be the main factor to trigger prostate cell growth.
Risk factors for benign prostatic hyperplasia (BPH)
There are many risk factors for benign prostatic hyperplasia. Men who are at a higher risk include:
- Age: men over the age of 50 are at a risk of BPH as it rises with age,
- Family history,
- Overweight or obesity increases the risk of BPH,
- Too much body fat may increase hormone levels and other factors in the blood, and stimulate the growth of prostate cells,
- Exercise can lower your risk of BPH,
- Men who don’t do exercise and stay active,
- Men at high risk who have metabolic syndrome, diabetes, high blood pressure and eat diet low in fruit, vegetables and legumes,
- Some men with erectile dysfunction (ED).
Prevention of BPH
There is no sure way to prevent BPH. But following lifestyle can help to prevent benign prostatic hyperplasia.
- Losing weight,
- Eating a well-balanced diet, rich in fruits and vegetables,
- Staying active also helps control weight and hormone levels.
Diagnosis
Consult your doctor if you have possible symptoms of benign prostatic hyperplasia. But immediately consult your doctor if you see that you have following symptoms:
- Blood in your urine,
- Pain or burning while passing urine,
- Inability to urinate.
